Ammonia production
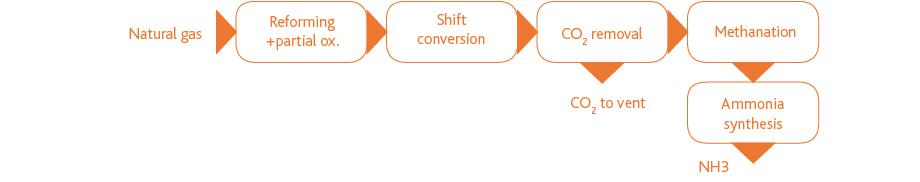
In natural gas-based ammonia production, steam-reforming and partial-oxidation technology are used to convert natural gas into syngas consisting mainly of hydrogen and nitrogen. The carbon present in the natural gas feedstock is mainly converted to CO and CO2. The syngas is then processed in a shift reactor to convert CO into CO2, which generates additional hydrogen. Complete removal of CO and CO2 is required as they both are poisons for the ammonia synthesis catalyst.
Processes:
Products:
Requirements:
The removal of CO2 downstream of the shift reactortypically involves an amine-based acid gas removal process. The final purification step is a catalytic methanation bed to convert any residual CO or CO2 into methane, an inert gas in the ammonia synthesis loop.
Challenges
Selection of the optimal solvent and plant design for the CO2 removal unit is a balancing exercise between CO2 removal rate, energy consumption, and overall plant cost. Typically, the gas to the methanator contains 500 to 1000 ppmv of residual CO2. Due to plant optimization and energy integration, a limited amount of heat is available within the complex to drive the amine regeneration. In addition to the correct selection of amine solvent, the plant design is also geared toward minimal energy consumption through the application of flash regeneration on the bulk portion of the amine solvent.
The CO2 stream is either vented or reutilized for the production of urea, in which ammonia is a precursor. Where urea production is concerned, it is desirable to maximize the pressure of the CO2 stream, which again impacts plant design and solvent selection.
Eastman’s answer
Eastman AdapT solvents are formulated to achieve targeted CO2 removal while minimizing energy consumption.
Eastman’s technical services team can help in optimizing plant design. Learn more about our technical services here.