Natural gas processing
Natural gas extracted from the earth may contain, in addition to valuable hydrocarbon components, undesirable contaminants such as water, mercury, carbon dioxide (CO2), hydrogen sulfide (H2S), mercaptans, and carbonyl sulfide (COS). CO2, H2S, mercaptans, and COS are generally termed acid gases because they form acids in aqueous solutions. These contaminants need to be removed, to varying degrees, according to the target use for the gas e.g., LNG production, pipeline transportation, or local consumption for power production. In addition, some gas wells contain sulfur species that also need to be removed.
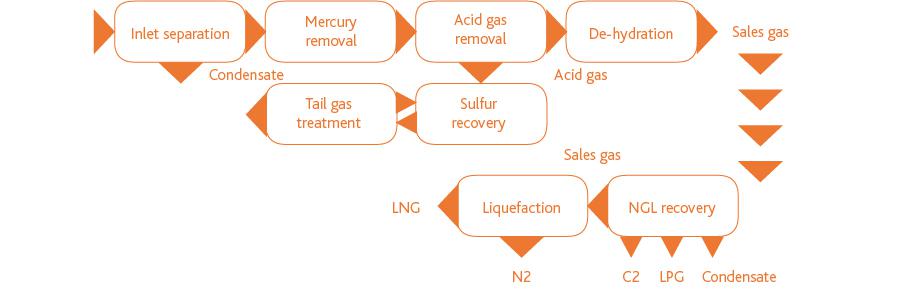
Processes:
Products:
Requirements
- CO2: The specifications for CO2 removal vary according to the target use:
- LNG (liquefied natural gas) production: Stringent specifications due to risk of solidification in the cold box
- Pipeline transportation: Generally up to 2 percent of CO2 is permitted—a limit which is associated with pipeline corrosion, gas calorific value, and international gas standards.
-
Local consumption of natural gas for power production: higher CO2 concentrations can be accepted because downstream equipment can be adapted to accommodate the higher concentrations.
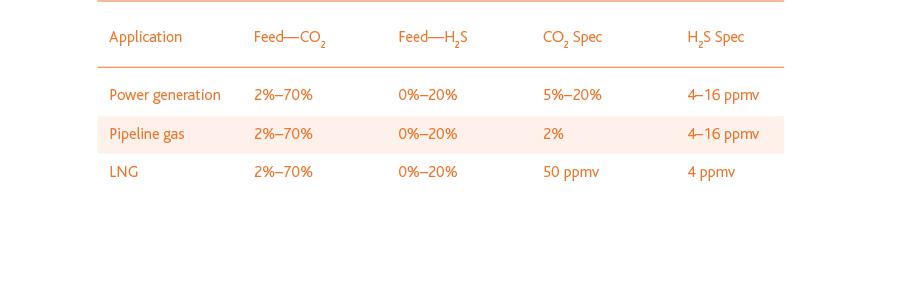
- H2S: Sulfur must be reduced to very low concentrations for reasons of safety, corrosion and environmental impact.
- Mercaptans and COS: These components can also be removed with amine solvents. Their removal is more challenging because they are very weak acids and hence react less strongly. These cases need to be evaluated on an individual basis. The optimal solution may be a combination of amine with another acid gas removal technology.
Considering pressure & temperature
Pressure: The pressure of the natural gas stream at the inlet of the gas treatment facility is usually high to very high. This is beneficial for the acid gas removal as well as other treatment steps. High pressure enables higher amine solvent loading, lower regeneration requirements, and smaller equipment design.
Temperature: Care must be taken not to exceed temperature limits within the absorber, where the temperature bulge (maximum temperature inside the absorber) can be much higher than the apparent absorption temperature measured in the treated gas and rich amine solvent stream.
Challenges
For optimal solvent selection, the pressure and temperature aspects should also be considered. This requires significant know-how and design experience.
Eastman’s answer
Eastman has a global network of technical experts that can help customers select the right solvent for their facility. For support on your individual situation, please contact us.